publications, Réseau
Instability of Cobalt-Substituted Polyoxometalates during the Oxygen Evolution Reaction: An Operando X-ray Absorption Spectroscopy Study
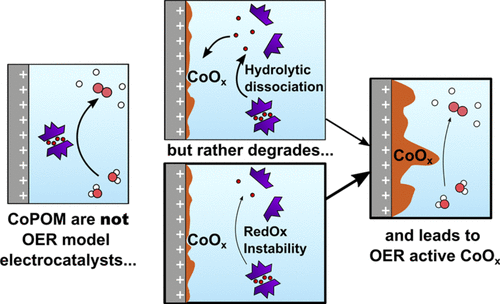
Complexes of cobalt(II) stabilized by lacunary polyoxometalates (CoPOMs) are highly discussed water oxidation catalysts (WOC). While their activity and stability in the oxygen evolution reaction (OER) have been widely explored, there is still no consensus between those claiming that CoPOMs are active and stable OER catalysts and those suggesting that they rather act as precatalysts, which degrade into OER-active heterogeneous CoOx catalysts. In this work, we use operando X-ray absorption spectroscopy along with electrochemical methods (cyclic voltammetry, chronoamperometry) to assess the activity and stability of [Co9(H2O)6(OH)3(HPO4)2(PW9O34)3]16– (Co9POM) under chemical and electrochemical operating conditions. First, we demonstrate that Co9POM dissolved in a phosphate buffer quickly degrades during an electrochemical OER, leaving a Co(III)/Co(II)-containing layer on the electrode surface that acts as a heterogeneous OER catalyst. This degradation is detected using both the post mortem and operando X-ray absorption near-edge structure and extended X-ray absorption fine structure. Then, the electrochemical OER is studied in the presence of 2,2′-bipyridine, which is used to eliminate Co2+ aqua-complexes resulting from Co9POM dissociation equilibrium. Yet, this does not avoid the formation of a Co-containing precipitate, albeit of a different composition. Finally, to differentiate between degradation associated with the catalytic cycle itself and the one provoked by local pH changes in the vicinity of the electrode during the OER, the Co9POM is studied as a homogeneous WOC with NaClO as a chemical oxidant. An irreversible loss of Co from the Co9POM is detected after the addition of two NaClO equivalents per Co ion. These combined insights provide clear operando evidence of Co9POM instability under either electrochemical OER or chemical WOC operating conditions.
B. Rotonnelli, Be. Lassalle-Kaiser, A. Bonnefont, S. Renaudineau, D. Garnier, A. Proust, F. Bournel, J.-J. Gallet, T. Asset, and E. Savinova
J. Phys. Chem. C 2024, 128, 19, 7968–7976; https://doi.org/10.1021/acs.jpcc.4c01265
Comments are closed